ACID/BASE ACTIVATION
STIMULATED RELEASE OF SIZE–SELECTED CARGOS IN SUCCESSION FROM MESOPOROUS SILICA NANOPARTICLES.
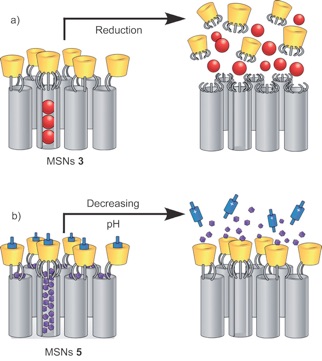
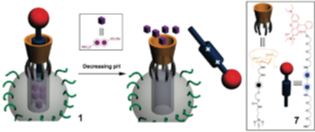
Covalent attachment of β-cyclodextrin rings (yellow; see figure) to mesoporous silica nanoparticles (MSNs) results in a dual-cargo release system into which differently sized cargos (blue and red spheres) can be loaded and then released in sequence when triggered by two different stimuli.
PH-OPERATED NANOPISTONS ON THE SURFACES OF MESOPOROUS SILICA NANOPARTICLES.
The supramolecular machine behaves like a nanopiston, releasing encapsulated guest molecules in a controlled fashion when the pH is lowered to ∼5.
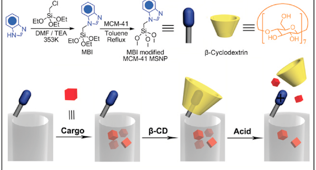
The N-methylbenzimidazole stalks with the optimized pKa endow the nanovalves with the capability of binding the CD ring strongly at pH 7.4 and trapping dye and drug molecules in the nanopores. Deprotonation at pH <6, as in acidifying endosomal compartments, causes dissociation of the -CD caps and release of the cargo molecules.
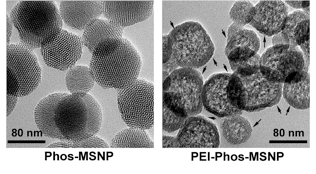
Since the polymer attachment leaves the porous interior free for drug binding and delivery, it establishes the potential to achieve simultaneous drug and nucleic acid delivery.
CONTROLLED-ACCESS HOLLOW MECHANIZED SILICA NANOCONTAINERS.
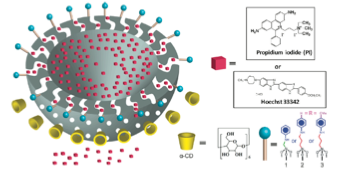
When the α -CD ring is complexed with the stalk at neutral pH, the bulky cyclic component is located near the pore openings, thereby blocking departure of cargo molecules that were loaded in the nanopores and hollow interior of the particle. Protonation of the nitrogen atoms at lower pH causes the binding affinity to decrease, releasing the α-CD and allowing the cargo molecules to escape.
PH-RESPONSIVE MECHANISED NANOPARTICLES GATED BY SEMIROTAXANES.
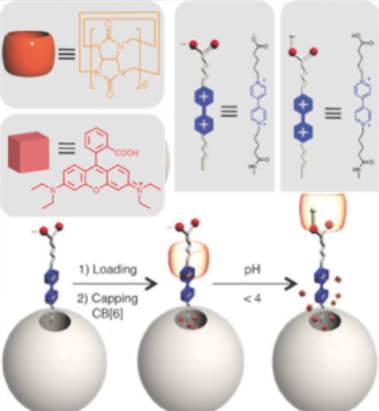
This [2]pseudorotaxane-based mechanised nanoparticle system, affords both water-soluble stalk and ring components in an effort to improve the biocompatibility of these promising new drug delivery vehicles.
PH-RESPONSIVE MECHANISED NANOPARTICLES GATED BY SEMIROTAXANES.
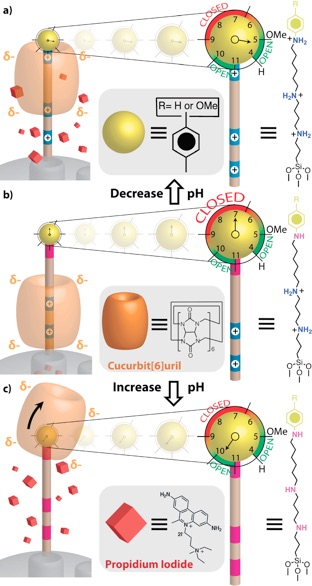
When the pH is lowered such that the anilinium nitrogen atom is protonated, the CB[6] ring shuttles to the distal hexa- methylenediammonium station, and cargo is released. At neutral pH, the CB[6] ring sits on the tetramethylenediammonium recognition unit, blocking the nanopores. When the pH is raised, all of the nitrogen atoms on the stalk are deprotonated resulting in dethreading of the CB[6] ring.
DUAL-CONTROLLED NANOPARTICLES EXHIBITING AND LOGIC.
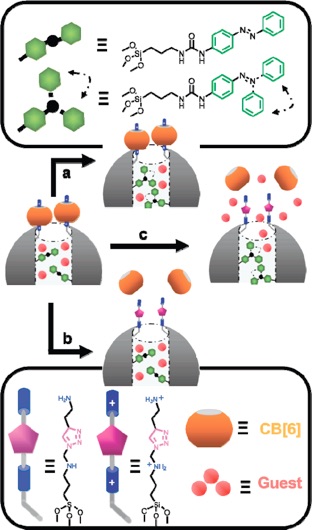
Excitation with 448 nm light induces the dynamic wagging motion of the nanoimpellers, but the nanovalves remain shut and the contents are contained. Addition of NaOH opens the nanovalves, but the static nanoimpellers are able to keep the contents contained. Simultaneous excitation with 448 nm light AND addition of NaOH causes the contents to be released.
REDOX- AND PH-CONTROLLED MECHANIZED NANOPARTICLES.
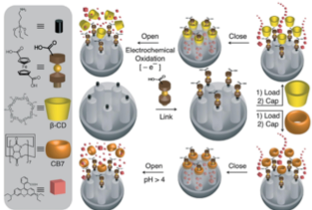
The system operated successfully under pH control (deprotonation of ferrocenedicarboxylic acid at pH = 4) in the presence of cucubit[7]uril, and redox control (oxidation of ferrocenedicarboxylic acid) in the presence of β-CD.
PH-RESPONSIVE SUPRAMOLECULAR NANOVALVES BASED ON CUCURBIT[6]URIL PSEUDOROTAXANES.
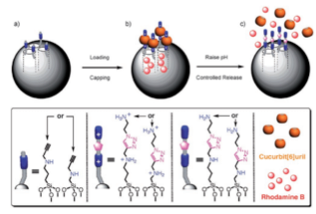
The alkyne-functionalized mesoporous silica nanoparticles are loaded with dye (rhodamine B) molecules, and capped with CB[6]. Cargo is released by switching off the ion–dipole interactions between the CB[6] rings and the bis ammonium stalks upon raising the pH value.
SUPRAMOLECULAR NANOVALVES CONTROLLED BY PROTON ABSTRACTION AND COMPETITIVE BINDING.
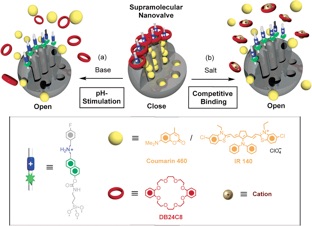
The versatility of supramolecular chemistry has been exploited in constructing nanovalves based on mesoporous silica MCM-41 and the mutual recognition between secondary dialkylammonium ions and dibenzo[2654]crown-8 (DB24C8). Naphthalene-containing dialkylammonium threads were tethered to the MCM-41, followed by loading with coumarin 460 and capping with DB24C8.